Study Title
Protocol for the AOAC International
Use-Dilution Methods (955.14, 955.15, & 964.02)
Product Identity
BIODOX CONCENTRATED LIQUID STERILIZER
Data Requirement
40 CFR PART 158—DATA REQUIREMENTS FOR PESTICIDES Subpart W—Antimicrobial Pesticides Guideline No. 810.2200
Author
Taylor Dreves Microbiologist II
Study Completion Date
10-26-2021
Testing Facility
Q Laboratories 1930 Radcliff Drive Cincinnati, OH 45204 (513) 471-1300
Laboratory Project Number (Study File)
QL 37018
1.0 EFFICACY STUDY SUMMARY
STUDY TITLE:
Protocol for the AOAC International Use-Dilution Methods (955.14,955.15, & 964.02)
LABORATORY PROJECT#:
QL 370181
GUIDELINE:
Guideline No. 810.2200 using Official Methods of Analysis of the AOAC International, Chapter 6, Disinfectants, Use-Dilution Methods (955.14, 955.15, & 964.02). Current edition. AOAC International, 2275 Research Blvd., Suite 300, Rockville, MD 20850 [Section 14.1,14.2, and 14.3 of Appendix 1].
TESTING FACILITY:
Q Laboratories 1930 Radcliff Drive Cincinnati, OH 45204
STUDY DATES:
STUDY INITIATION DATE:
06-09-2021
STUDY COMPLETION DATE:
10-26-2021
GLP COMPLIANCE:
Q Laboratories has developed and implemented a quality management system that enhances our ability to provide testing services that consistently meet client expectations and regulatory requirements. All testing was performed in accordance with EPA Good Laboratory Practice Standards (GLPS), as specified in 40 CFR Part 160. Periodic phase audits of the study were conducted by the Quality Assurance Unit to ensure testing compliance and a review of the final report by the QAU was conducted in accordance with 40 CFR,Part 160.35, subpart B.
TEST SUBSTANCE:
DESCRIPTION:
BioDox CONCENTRATED LIQUID STERILIZER
% ACTIVE:
Chlorine Dioxide (CIO2), 0.4 %
INGREDIENT:
½ oz or 15 mL or 1 tbsp of substance to 32 oz (946 mL) water
DILUTION:
TEST CONDITIONS: 5% fetal bovine serum
SOIL LOAD: Test substance is diluted in AOAC hard water solution
WATER: prepared according to EPA SOP MB-30-02 [Section14.4 of Appendix 1] to use-dilution.
CONTACT TIME: 3 minutes ± 5 seconds
TEMPERATURE: Ambient Temperature (20 – 25 °C)
OTHER: The inoculum applied includes 5% fetal bovine serum.
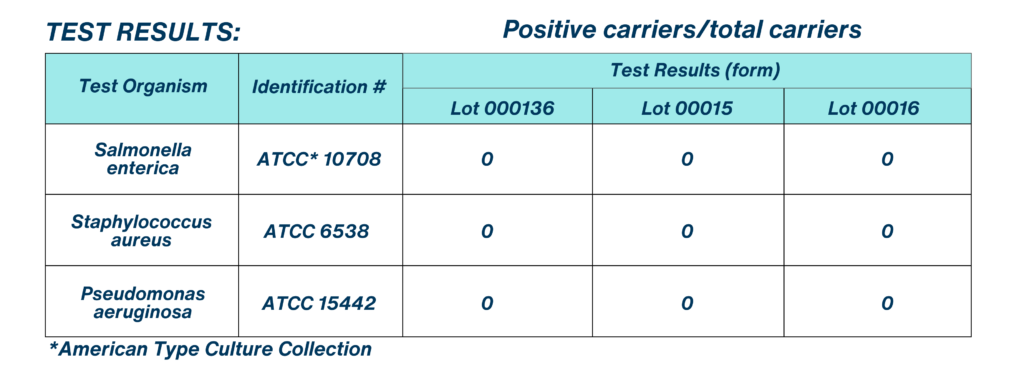
CONTROL RESULTS:
The control carriers for S. aureus and P. aeruginosa were between 1.0 x 106 to 1.0 x 107 CFU/carrier. The control carriers for S. enterica were between 1.0 x 105 to 1.0 x 106 CFU/carrier. Growth occurred in all viability control tubes. Growth did not occur in any of the sterility tubes. Neutralization was considered adequate and meet the specification in Section 13.0 of Appendix 1. For media quality controls, comparable growth acceptance was within 50 – 200 %. No growth occurred in the media sterility control. No disrupted pellicles of P. aeruginosa test culture were used. No contamination occurred in the subculture tubes.
CONCLUSION:
Based on the results presented in this study report, the test article met the performance standard: (0) positive carriers out of sixty (60) for each lot, when tested against Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella enterica. The performance standard listed in 810.2200 for S. aureus and P. aeruginosa is no more than three positive carriers out of 60 per test. The performance standard for S. enterica is no more than one positive carrier out of 60 per test. All testing was performed in accordance with EPA Good Laboratory Practice Standards (GLPS), as specified in 40 CFR Part 160. Periodic phase audits of the study were conducted by the Quality Assurance Unit to ensure testing compliance and a review of the final report by the QAU was conducted in accordance with 40 CFR, Part 160.35, subpart B. For more information regarding this Efficacy Study with Q Labs, please contact us and we would love to share more of our amazing results.

BioDox™ can be used on high value Equipment and will leave the least amount of corrosion.
BioDox™ ideal for high-value equipment, leaving minimal corrosion, residues, and preventing resistance. Safeguard your assets while ensuring optimal performance and protection.
PPM Strength: 25 – 50ppm
Ratio: 1oz to 2oz per gallon
Directions:
Mix in spray bottle and apply directly to equipment. BioDox leaves no residue and does not need to be rinsed. Another option is by immersion for 10 minutes.
Benefits:
BioDox is the most effective solution for your sterilization needs because it destroys up to 99.9999% of pathogens. It is effective against the most dangerous germs and destroys the most dangerous biofilm. BioDox will leave the least amount of corrioson on your high value equipment.
Apply BioDox™ wherever mold or biofilm is present, including fans and operational fixtures, for effective remediation and cleanliness.
PPM Strength: 50 – 100ppm
Ratio: 2oz per gallon or 4oz per gallon
Directions:
Mix in spray bottle and apply directly to equipment. BioDox leaves no residue and does not need to be rinsed. Another option is by immersion for 10 minutes.
Benefits:
BioDox is the most effective solution for your sterilization needs because it destroys up to 99.9999% of pathogens. It is effective against the most dangerous germs and destroys the most dangerous biofilm. BioDox will leave the least amount of corrioson on your high value equipment.
BioDox™ provides biofilm Control in Irrigation tanks, lines and emitters.
BioDox™ excels in biofilm control in irrigation systems, safely eliminating pathogens. It also cleans tanks, lines, and emitters. Ideal for use at the start of the growing season and when termperatures begin to rise in the summer.
PPM Strength: 5ppm
Ratio: 2oz per 10 gallons
Directions:
Biodox is added to the main irrigation tanks at a rate of 2oz per ten gallons. It is best to use at the start of the growing season and when temperatures begin to rise in the summer.
Benefits:
BioDox destroys the most dangerous biofilm in irrigation tanks and lines. It is the best chemistry for killing biofilm without toxic side-effects.
BioDox™ can be used on all other surfaces including walls, floors, cabinets, containers, sinks and countertops.
Safeguard your surroundings from pathogens with BioDox™. Versatile across all surfaces: walls, floors, cabinets, sinks, and countertops. Ensure a clean and pathogen-free environment.
PPM Strength: 50 – 100ppm
Ratio: 2oz per gallon or 4oz per gallon
Directions:
Mix in spray bottle and apply directly as fine mist. BioDox can be applied on all surfacas including walls, floors, cabinets, sinks and countertops. It can be used to steriilize your tools as well. BioDox is considered non-toxic and leaves no residue. No rinsing is needed.
Benefits:
BioDox can be used anywhere and on anything to promote a sterile environment. BioDox destroys up to 99.9999% of harmful pathogens and it also destroys the most dangerous biofilm. BioDox replaces Bleach in any application.
BioDox™ is effective on skin, hands, clothing, boots, and shoes. Crucial for self-sterilization to prevent pathogen spread when moving between rooms or plants.
PPM Strength: 50 – 100ppm
Ratio: 2oz per gallon or 4oz per gallon
Directions:
Mix in spray bottle and apply directly as fine mist. Spray down your shoes, your clothing, and directly on to your hands and skin. BioDox is considered non-toxic and leaves no residue. No rinsing is needed.
Benefits:
BioDox is effective against the most resistant pathogens. It does not dry out the skin or leave any toxic residues. It is a great resource to use when traveling between rooms, crops & greenhouses to ensure you do not cross contaminate. ***On clothes, do not apply if tincture is synthetic as it may discolor.
BioDox™ is a Foodwash: FDA GRAS (Generally Recognized as Safe) and offers benefits like pathogen removal, reduced pesticide residues, extended shelf life, improved food safety, enhanced quality, and peace of mind.
PPM Strength: 3 – 5ppm
Ratio: 3 – 6ml per gallon
Directions:
Calculate final ppm for immersion in water. First fill with water, then add Biodox, then immerse the food for 5 to 10 minutes.
Benefits:
BioDox is a Chlorine Dioxide which is FDA GRAS (Generally recognized as safe). BioDox destroys 99.9999% of pathogens, including amoebas, E. Coli, Salmonella, Norovirus, Toxoplama gondi, Staphylococcus aureus, Campylobacter, among other food-born bacteria causing food poisoning and hospitalizations
BioDox can also be used as a root drench after plants are in the ground.
Root Drench with Biodox treats soil, reducing pathogen colonies without harming beneficial microbes or causing lockout. Fosters a healthy environment for beneficial microbes to thrive.
PPM Strength: 2.5 – 5ppm
Ratio: 1oz per 10 gallons to 2oz per 10 gallons
Directions:
For preventive maintenance use a 2.5ppm (1oz per ten gallons) solution every other week throughout Veg and the first six weeks of flowering. If there is an infection, use Biodox at a 5 ppm solution (2oz per ten gallons) every week until symptoms subside and then every other week until harvest. Apply product through the watering system during the watering cycle between feedings. Allow the soil to dry back as much as possible until plants begin to shows signs of wilt, then resume watering and feeding as usual.
Benefits:
Root Drench is a soil treatment with Biodox performed while the plant is in the soil. The root drench method allows for colonies of pathogens to be reduced without destroying good microbes or causing lock out. This allows the beneficial microbes an opportunity to dominate the terrain. The root drench method allows for colonies of pathogens to be reduced without destroying good microbes or causing lock out. This allows the beneficial microbes an opportunity to dominate the terrain.
BioDox™ can be used to Sterilize the soil at 25ppm before planted.
Soil sterilization is essential for pathogen control and biofilm removal, benefiting the entire water system. Best done at the season’s start or between harvests for plant health.
PPM Strength: 25ppm
Ratio: 1oz per gallon
Directions:
25ppm solution of Biodox in the water system for the farm. This solution travels from the water tank through the pipes and emitters to then fully saturates the soil. Depending on conditions, 60-80 gallons per yard is applied and allowed to completely dry back. It is recommended to allow the product to dissipate for three days before introducing new plants into the soil.
Benefits:
Soil Sterilization is a critical step to ensure that colonies of pathogens are reduced or eliminated before the plants are introduced to the soil. Additional benefits of this approach include cleaning the tank, lines and emitters of biofilm. Soil Sterilization is recommended at the beginning of the growing season, or between harvesting and planting the next round.
Biodox foliar application enhances plant health, increases yields and terpenes, while selectively targeting and oxidizing pests without toxicity. It’s non-toxic and suitable for late flowering stages.
PPM Strength: 25 – 50ppm
Ratio: 1oz to 2oz per gallon
Directions: 1 oz per gallon (25ppm) or 2oz per gallon (50ppm)
Benefits: Foliar applications are critical to maintain a sterile environment. Third party studies show that using Biodox as a plant wash removes biofilm from the leaves allowing for greater photosynthesis, creating higher yields and terpenes. Most importantly, Biodox targets pests like PM, Botrytis, and many other agricultural pathogens by selectively oxidizing them in a way no other chemical does. It discourages and oxidizes small pests like mites, aphids and thrips without toxicity or residue. Biodox can be used during the curing phase after harvest to discourage spider mites or pm without reducing THC or terpene content. Biodox is completely non-toxic and made of compounds not tested for in DCC testing, making it ideal for the last weeks of flowering.