Virucidal Efficacy of a Test Substance for Use on Inanimate, Nonporous Surfaces
BIODOX Chlorine Dioxide 4000 ppm
Lot Numbers: 000136, 21.05.19.01
Human coronavirus, 229E strain, ATCC VR-740
U.S. EPA OCSPP 810.2200
Madhuri Patil, M.S.
Sept. 20 2021
Microchem Laboratory 1304 W. Industrial Blvd Round Rock, Texas 78681
BioCentric Solutions
12400 Loma Rica Dr
Grass Valley, CA 95945
Study Title: Virucidal Efficacy of a Test Substance for Use on Inanimate Nonporous Surfaces
Study Identification Number: GLP2845
Test Microorganism: Human coronavirus, 229E strain, ATCC VR-740
Host Cell: MRC-5 cells (ATCC CCL-171)
Test Substance: BIODOX Chlorine Dioxide 4000 ppm
Lot Numbers: 000136,21.05.19.01
Test Substance Dilution: Diluted 30 ml test substance into 970 ml of 200 ppm autoclave-sterilized tap water
Test Substance Application: 2.0 ml aliquot of the use dilution of the liquid test substance applied via pipette
Organic Soil Load: No additional supplementation of organic soil load incorporated into the test inoculum
Inoculum Volume: 0.200 ml
Carrier Type: Sterile glass Petri dish (100 mm x 15 mm)
Number of Carriers per Lat: One
Contact Time: 10 minutes
Exposure Temperature: Ambient room temperature (24.0-24.6 C) and 31% relative humidity (RH)
Neutralization Method: Sephadex LH – 20 gel filtration column
Study Initiation Date: 04AUG2021
Experimental Start Date/Time: 05AUG2021 / 1455
Experimental End Date/Time: 12AUG2021 / 0911
Study Completion Date: 20SEP2021
Name: BioDox Chlorine Dioxide 4000 ppm
Lot: 000136
Active Ingredients (concentration): Chlorine dioxide (0.4%)*
Date of Manufacture: 10APR2021
Date Received: 04MAY2021
Expiration Date: 10NOV2021*
Lot: 21.05.19.01
Active Ingredients (concentration): Chlorine dioxide (0.4%)*
Date of Manufacture: 19 MAY 2021
Date Received: 01 JUN 2021
Expiration Date: 19 MAY 2022
*As indicated in the approved protocol.
Form: Liquid; dilution required.
Storage Conditions: Ambient room temperature under fluorescent lighting.
Test Substance Preparation: The test substance was used as directed by the Study Sponsor. Each lot of the test substance was prepared by adding 30ml of test substance 970ml of autoclave sterilized tap water. The prepared test substance appeared to be in solution as determined by visual observation on the day of use.
The 200 ppm autoclave – sterilized tap water (180 – 210 ppm range) used as the test substance diluent was titrated using a calibrated buret on the day of use. The titration result was 182 ppm.
The test substance was equilibrated to the requested exposure temperature prior to use.
Protocol Amendment #1
On 20SEP2021, the approved/signed protocol P3254 has been amended to reflect that the Certificates of Analysis for each lot of test substance will not be provided.
All remaining testing parameters are to be followed as stated in the protocol.
There were no deviations from the approved protocol during the conduct of this study.
The test substance was applied to a film of virus that has been dried onto the surface of a glass carrier (representing a hard, nonporous surface) and held for the Sponsor-specified contact time. At the conclusion of the contact time, the recovered virus-test substance mixture was neutralized and the mixture was assayed for infectivity. Plate recovery, cytotoxicity, neutralization, virus inoculum titer and cell culture controls are performed concurrently with the test.
Human coronavirus, 229E strain, ATCC VR-740, originally received from the American Type Culture Collection (ATCC), Manassas, VA, was used in this study. The Microchem Laboratory lot number used in testing was HCoV_21MAR2020C.
ATCC microorganisms are used under commercial license. The ATCC trademark and trade name and any and all ATCC catalog numbers are trademarks of the American Type Culture Collection.
The test virus was propagated internally by Microchem Laboratory personnel by inoculating the virus into cell culture flasks containing the appropriate host cell line and incubating at the appropriate conditions. Once the cell cuore flasks displayed approximately 75-100% cytopathic effect (as determined by microscopic evaluation), the flasks were subjected to freeze-thaw cycles to release virus from infected cells. The contents of the cell culture flasks were collected and centrifuged in order to remove the cell debris. The test virus was then aliquoted and stored at s- 70 °C. On the day of testing, the appropriate number of virus stock suspension vials were removed from cryostorage and thawed for use in the assay. The test virus contained 2% fetal bovine serum (FBS) organic soil load. The test virus was not adjusted to incorporate any additional organic soil load into the inoculum.
MRC-5 cells (ATCC CCL-171), originally received from the ATCC, were utilized in the assay. The cells were subcultured by Microchem Laboratory personnel and seeded into 24-well cell culture plates. The plates were incubated at 36±2°C in a humidified atmosphere of 6±1% CO₂ until they reached the desired confluence required for testing. On the day of use, the cells were microscopically examined to verify the appropriate confluency and health of the cells. Cell culture passage documentation including cell culture source, passage number, seeding densities, etc. was retained.
ATCC microorganisms are used under commercial license.
The ATCC trademark and trade name and any and all ATCC catalog numbers are trademarks of the American Type Culture Collection.
The test medium utilized in the assay was Eagle’s Minimum Essential Medium (EMEM) supplemented with 2% FBS, 40 mM HEPES buffer, 125 µM non-essential amino acids, 1 mM sodium pyruvate, plus antibiotics [antibiotic-antimycotic solution (100 units/ml penicillin G, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B)]. Concentrations based on preparation of 1 L of Eagle’s Minimal Essential Medium.
The test substance was used as directed by the Study Sponsor. Each lot of the test substance was prepared by adding 30 ml of test substance to 970 ml of autoclave-sterilized tap water. The prepared test substance appeared to be in solution as determined by visual observation on the day of use. The 200 ppm autoclave-sterilized tap water (180-210 ppm range) used as the test substance diluent was titrated using a calibrated buret on the day of use. The titration result was 182 ppm. The test substance was equilibrated to the requested exposure temperature prior to use.
Sephadex LH-20 gel filtration columns were utilized to neutralize and/or to reduce the cytotoxicity of the test substance following exposure to the test virus by separating the virus from the test substance via filtration. On the day of testing, the prepared Sephadex slurry was aseptically added to prepared column units (sterile syringe) to completely fill the column. Just prior to testing, the syringe was centrifuged at approximately 100 x g for 3-4 minutes to clear the void volume.
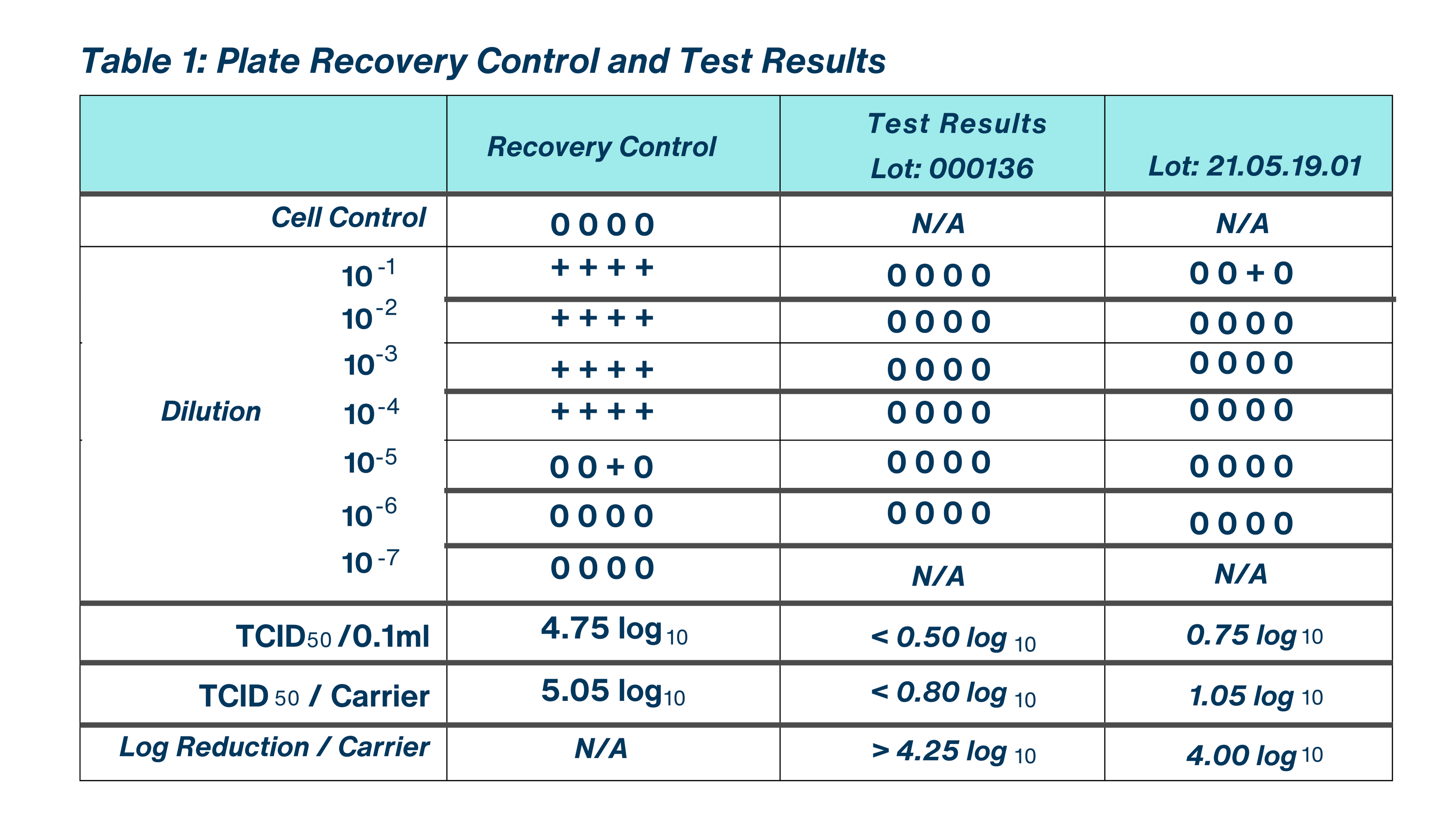
The test virus was vortexed thoroughly and a 0.200 ml aliquot of virus was placed on the inside bottom surface of three 100 mm x 15 mm sterile glass Petri dishes which served as the test carriers and plate recovery control. The inoculum was spread over the entire area of the carriers using a sterile bent pipette tip without touching the sides of the Petri dish. The virus films were dried in an environmental chamber for 20 minutes at 20.0 °C in a relative humidity of 30%. Exposure of Virus Films to the Test Substance For each lot of the test substance, one dried virus film carrier was treated with a 2.0 ml aliquot of the use dilution of the liquid test substance following Study Sponsor instructions. The carriers were gently rotated to ensure complete coverage of the test substance over the entirety of each test surface. The carriers were held at the Sponsor-requested exposure temperature of 24.0-24.6 °C in a relative humidity of 31% for the requested contact time of 10 minutes. Just prior to the completion of the contact time, a sterile cell scraper was used to re-suspend each viral film and the solution was immediately transferred into a gel filtration column. The syringe plunger was used to pass the contents of the re-suspended test carrier through the column. Serial 10-fold dilutions (e.g. 0.1 ml filtrate + 0.9 ml test media) of the filtrate (10 dilution) were prepared to the appropriate dilution.
One plate recovery control film was prepared to determine the baseline dried virus titer. The plate recovery control film was generated as described above in “Preparation of Virus Films.” Following drying, a 2.0 ml aliquot of test medium was overlaid on the control film. The carrier was then gently rotated to ensure complete coverage of the solution over the entirety of the surface. The carrier was held covered at the Sponsor-requested exposure temperature of 24.1- 24.4 °C in a relative humidity of 31% for the requested contact time of 10 minutes. Just prior to the completion of the study contact time, a sterile cell scraper was used to re-suspend each viral film and the solution was immediately transferred into a gel filtration column. The re-suspended content of the carrier was passed through the gel filtration column using the syringe plunger. Serial 10-fold dilutions (e.g. 0.1 ml filtrate + 0.9 ml test media) of the filtrate (10) dilution) were prepared to the appropriate dilution. The results of the plate recovery control were microscopically evaluated at the same time as the results of the test substance and all other controls. The results of this control were used to calculate the log reduction in viral titer following exposure of the test substance to the test virus.
For each lot of test substance assayed, one sterile glass Petri dish carrier (containing no virus film) was treated in the same manner as the test carriers. A 2.0 ml aliquot of the use dilution of the use dilution of the test substance was added to the sterile Petri dish and held covered at the Sponsor-requested contact time of 10 minutes at the requested exposure temperature of 23.9- 24.5 °C in a relative humidity of 30-31%. Just prior to the completion of the study contact time, the carrier was scraped using a sterile cell scraper, and the test substance suspension was promptly transferred into a gel filtration column. The re-suspended test substance was passed through the gel filtration column using the syringe plunger. Serial 10-fold dilutions (e.g. 0.1 ml filtrate + 0.9 ml test media) of the filtrate (10 dilution) were prepared to the appropriate dilution. The results of the cytotoxicity control were microscopically evaluated at the same time as the results of the test substance and all other controls.
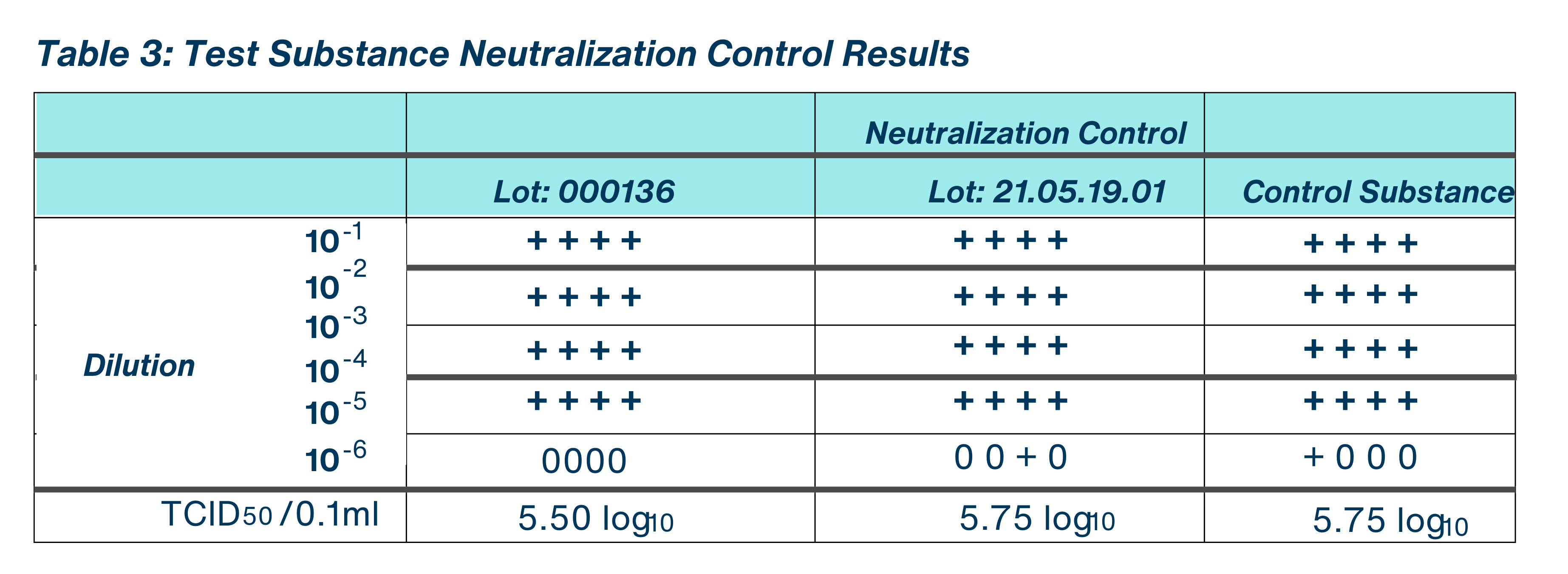
For each lot of test substance assayed, one sterile glass Petri dish carer (containing no virus film) was treated in the same manner as the test carriers. A 2.0 ml aliquot of the use dilution of the test substance was added to the sterile Petri dish. The carrier was scraped using a sterile cell scraper, and the test substance suspension was promptly transferred into a gel filtration column. The re-suspended test substance was passed through the gel filtration column using the syringe plunger. A 2.0 ml aliquot of test media, or other media as appropriate, was passed through the gel filtration column in the same manner as the test to serve as a neutralization control substance, to determine if comparable levels of infectious viral units were recovered from the control and the neutralized test substance filtrate. The filtrate is considered the 10′ dilution.
To verify that the test substance had been neutralized, the filtrate (neutralized test substance) and the neutralization control substance were each challenged with a 0.1 ml aliquot of low titer (e.g. 1000-5000) infective units of the test system and held for at least 10 minutes at an exposure temperature of 24.1-24.7 °C in a relative humidity of 30-31%. Serial 10-fold dilutions were prepared using test media by adding 0.100 ml filtrate to 0.900 ml test media. The results of the neutralization control were microscopically evaluated at the same time as the results of the test substance and all other controls.
To ensure that the host cells were not contaminated with bacteria, fungi, or any cytopathogenic viruses, and to confirm the viability of the cells during the incubation period of the assay, af least four cell monolayers were left untreated and microscopically examined periodically throughout the incubation period. Any obvious contamination or degeneration in such monolayers could invalidate the virucidal efficacy assay.
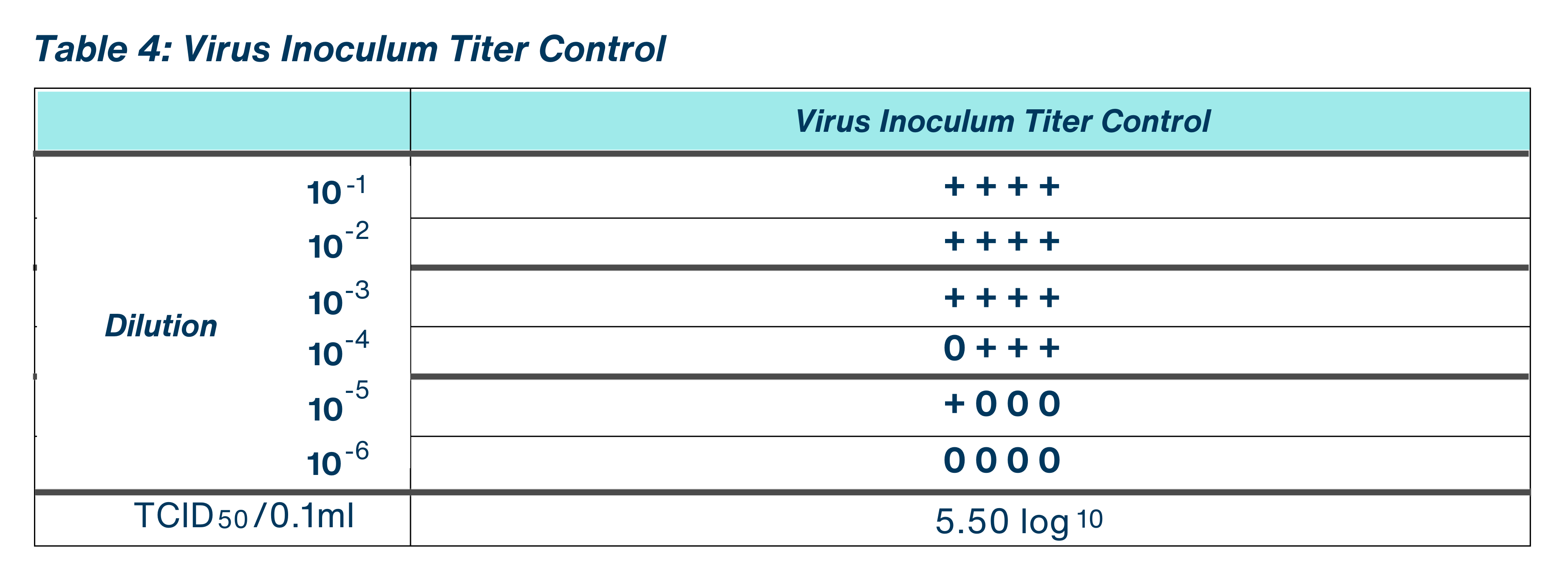
To confirm that the host cell-line monolayers were susceptible to the test virus and to confirm the titer of the viral inoculum, an aliquot of the test virus inoculum was serially diluted (10-fold) in test media. This control was also used to confirm the level of virus inoculated in the Neutralization Control.

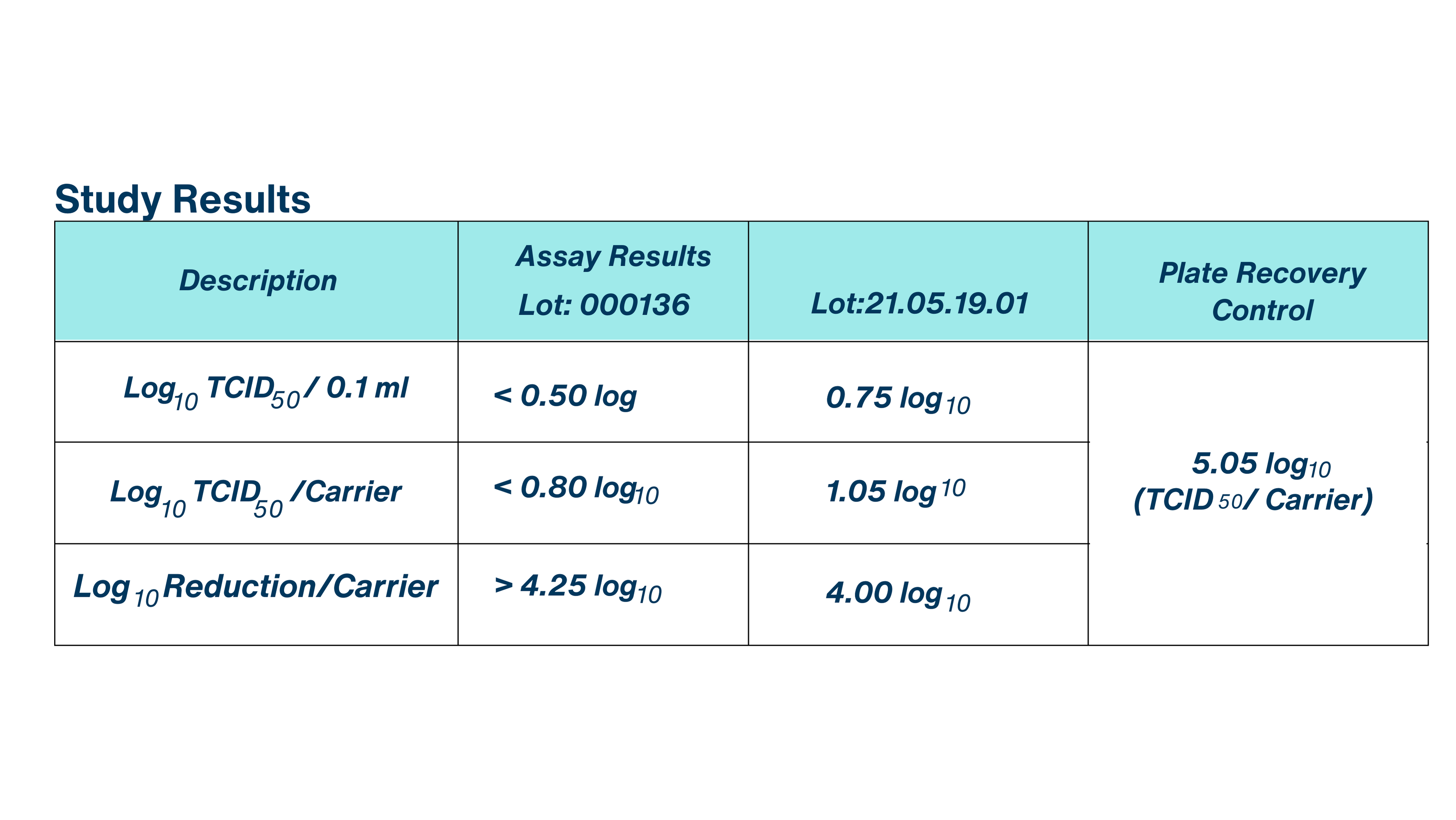
The purpose of the study was to determine the virucidal efficacy of BioDox Chlorine Dioxide 4000 ppm (Lots: 000136 and 21.05.19.01) against Human coronavirus, 229E strain, ATCC VR-740, with no additional supplementation of organic soil load incorporated into the test inoculum,at a contact time of 10 minutes and exposure temperature of room temperature.
The Plate Recovery Control demonstrated a viral titer of 4.75 log TCID per 0.1 ml and 5.05 log TCID per carrier, thereby satisfying U.S. EPA study acceptance criteria of a minimum of 4.80 log infective units per control carrier.
Taking the cytotoxicity and neutralization control results into consideration, the evaluated test substance, BioDox Chlorine Dioxide 4000 ppm, demonstrated a > 4.25 log reduction in viral titer for Lot: 000136 and a 4.00 log reduction in viral titer for Lot: 21.05.19.01. No test substance cytotoxicity was detected in either lot of test substance assayed, <0.50 log TCD per 0.1 ml for Lot: 000136 and < 0.5 log TCD per 0.1 ml for Lot: 21.05.19.01. The test substance and control substance demonstrated comparable levels of infective unitsrecovered in the Neutralization Control.
No microbial contamination of any host cell cultures was observed during the course of the study.
BioDox Chlorine Dioxide 4000 ppm (Lots: 000136 and 21.05.19.01) met the U.S. EPA Product Performance Guidelines for Disinfectants for Use on Hard Surfaces outlined in U.S. EPA OCSPP 810.2200 and the success criteria detailed in the approved protocol when tested against Human coronavirus, 229E strain, ATCC VR-740 at contact time of 10 minutes.
This study was carried out in compliance with the approved protocol. All experimental controls met the established acceptance criteria unless otherwise noted in the Protocol Changes section of this report. There were no circumstances that may have affected the quality or the integrity of the data.

BioDox™ can be used on high value Equipment and will leave the least amount of corrosion.
BioDox™ ideal for high-value equipment, leaving minimal corrosion, residues, and preventing resistance. Safeguard your assets while ensuring optimal performance and protection.
PPM Strength: 25 – 50ppm
Ratio: 1oz to 2oz per gallon
Directions:
Mix in spray bottle and apply directly to equipment. BioDox leaves no residue and does not need to be rinsed. Another option is by immersion for 10 minutes.
Benefits:
BioDox is the most effective solution for your sterilization needs because it destroys up to 99.9999% of pathogens. It is effective against the most dangerous germs and destroys the most dangerous biofilm. BioDox will leave the least amount of corrioson on your high value equipment.
Apply BioDox™ wherever mold or biofilm is present, including fans and operational fixtures, for effective remediation and cleanliness.
PPM Strength: 50 – 100ppm
Ratio: 2oz per gallon or 4oz per gallon
Directions:
Mix in spray bottle and apply directly to equipment. BioDox leaves no residue and does not need to be rinsed. Another option is by immersion for 10 minutes.
Benefits:
BioDox is the most effective solution for your sterilization needs because it destroys up to 99.9999% of pathogens. It is effective against the most dangerous germs and destroys the most dangerous biofilm. BioDox will leave the least amount of corrioson on your high value equipment.
BioDox™ provides biofilm Control in Irrigation tanks, lines and emitters.
BioDox™ excels in biofilm control in irrigation systems, safely eliminating pathogens. It also cleans tanks, lines, and emitters. Ideal for use at the start of the growing season and when termperatures begin to rise in the summer.
PPM Strength: 5ppm
Ratio: 2oz per 10 gallons
Directions:
Biodox is added to the main irrigation tanks at a rate of 2oz per ten gallons. It is best to use at the start of the growing season and when temperatures begin to rise in the summer.
Benefits:
BioDox destroys the most dangerous biofilm in irrigation tanks and lines. It is the best chemistry for killing biofilm without toxic side-effects.
BioDox™ can be used on all other surfaces including walls, floors, cabinets, containers, sinks and countertops.
Safeguard your surroundings from pathogens with BioDox™. Versatile across all surfaces: walls, floors, cabinets, sinks, and countertops. Ensure a clean and pathogen-free environment.
PPM Strength: 50 – 100ppm
Ratio: 2oz per gallon or 4oz per gallon
Directions:
Mix in spray bottle and apply directly as fine mist. BioDox can be applied on all surfacas including walls, floors, cabinets, sinks and countertops. It can be used to steriilize your tools as well. BioDox is considered non-toxic and leaves no residue. No rinsing is needed.
Benefits:
BioDox can be used anywhere and on anything to promote a sterile environment. BioDox destroys up to 99.9999% of harmful pathogens and it also destroys the most dangerous biofilm. BioDox replaces Bleach in any application.
BioDox™ is effective on skin, hands, clothing, boots, and shoes. Crucial for self-sterilization to prevent pathogen spread when moving between rooms or plants.
PPM Strength: 50 – 100ppm
Ratio: 2oz per gallon or 4oz per gallon
Directions:
Mix in spray bottle and apply directly as fine mist. Spray down your shoes, your clothing, and directly on to your hands and skin. BioDox is considered non-toxic and leaves no residue. No rinsing is needed.
Benefits:
BioDox is effective against the most resistant pathogens. It does not dry out the skin or leave any toxic residues. It is a great resource to use when traveling between rooms, crops & greenhouses to ensure you do not cross contaminate. ***On clothes, do not apply if tincture is synthetic as it may discolor.
BioDox™ is a Foodwash: FDA GRAS (Generally Recognized as Safe) and offers benefits like pathogen removal, reduced pesticide residues, extended shelf life, improved food safety, enhanced quality, and peace of mind.
PPM Strength: 3 – 5ppm
Ratio: 3 – 6ml per gallon
Directions:
Calculate final ppm for immersion in water. First fill with water, then add Biodox, then immerse the food for 5 to 10 minutes.
Benefits:
BioDox is a Chlorine Dioxide which is FDA GRAS (Generally recognized as safe). BioDox destroys 99.9999% of pathogens, including amoebas, E. Coli, Salmonella, Norovirus, Toxoplama gondi, Staphylococcus aureus, Campylobacter, among other food-born bacteria causing food poisoning and hospitalizations
BioDox can also be used as a root drench after plants are in the ground.
Root Drench with Biodox treats soil, reducing pathogen colonies without harming beneficial microbes or causing lockout. Fosters a healthy environment for beneficial microbes to thrive.
PPM Strength: 2.5 – 5ppm
Ratio: 1oz per 10 gallons to 2oz per 10 gallons
Directions:
For preventive maintenance use a 2.5ppm (1oz per ten gallons) solution every other week throughout Veg and the first six weeks of flowering. If there is an infection, use Biodox at a 5 ppm solution (2oz per ten gallons) every week until symptoms subside and then every other week until harvest. Apply product through the watering system during the watering cycle between feedings. Allow the soil to dry back as much as possible until plants begin to shows signs of wilt, then resume watering and feeding as usual.
Benefits:
Root Drench is a soil treatment with Biodox performed while the plant is in the soil. The root drench method allows for colonies of pathogens to be reduced without destroying good microbes or causing lock out. This allows the beneficial microbes an opportunity to dominate the terrain. The root drench method allows for colonies of pathogens to be reduced without destroying good microbes or causing lock out. This allows the beneficial microbes an opportunity to dominate the terrain.
BioDox™ can be used to Sterilize the soil at 25ppm before planted.
Soil sterilization is essential for pathogen control and biofilm removal, benefiting the entire water system. Best done at the season’s start or between harvests for plant health.
PPM Strength: 25ppm
Ratio: 1oz per gallon
Directions:
25ppm solution of Biodox in the water system for the farm. This solution travels from the water tank through the pipes and emitters to then fully saturates the soil. Depending on conditions, 60-80 gallons per yard is applied and allowed to completely dry back. It is recommended to allow the product to dissipate for three days before introducing new plants into the soil.
Benefits:
Soil Sterilization is a critical step to ensure that colonies of pathogens are reduced or eliminated before the plants are introduced to the soil. Additional benefits of this approach include cleaning the tank, lines and emitters of biofilm. Soil Sterilization is recommended at the beginning of the growing season, or between harvesting and planting the next round.
Biodox foliar application enhances plant health, increases yields and terpenes, while selectively targeting and oxidizing pests without toxicity. It’s non-toxic and suitable for late flowering stages.
PPM Strength: 25 – 50ppm
Ratio: 1oz to 2oz per gallon
Directions: 1 oz per gallon (25ppm) or 2oz per gallon (50ppm)
Benefits: Foliar applications are critical to maintain a sterile environment. Third party studies show that using Biodox as a plant wash removes biofilm from the leaves allowing for greater photosynthesis, creating higher yields and terpenes. Most importantly, Biodox targets pests like PM, Botrytis, and many other agricultural pathogens by selectively oxidizing them in a way no other chemical does. It discourages and oxidizes small pests like mites, aphids and thrips without toxicity or residue. Biodox can be used during the curing phase after harvest to discourage spider mites or pm without reducing THC or terpene content. Biodox is completely non-toxic and made of compounds not tested for in DCC testing, making it ideal for the last weeks of flowering.